Diet and senses

What did plesiosaurs eat and how do we know?
Types of evidence for diet
Pollard (1990) established three broad categories of evidence for diet in prehistoric organisms: direct evidence, indirect evidence and general evidence. Direct evidence consists of various grades of gut contents, indirect evidence includes bite marks, and general evidence consists of functional analyses. Focussing specifically on the Middle Jurassic Oxford Clay Formation, Martill et al. (1994) identified eight categories of evidence for determining trophic links:
1. Incontrovertible evidence. e.g. Gut contents, distinctive bite marks.
2. Close association of fossil finds. e.g. Pliosaur teeth near a fish skeleton.
3. Functional morphological analysis. e.g. Dental morphology, body size, hydrodynamic shape.
4. Proxy evidence as in 1 above from other deposits. Stomach contents in related taxa.
5. Comparison with extant homologues or analogues. e.g. Killer whale, great white shark, gavial, dolphin, sperm whale.
6. Geochemical data
7. Sedimentological/biostratinomic data
8. Supposed relationships
The following evidence provides information on diet in plesiosaurs. The superscript number refer to the types of evidence listed above.
Gut contents or ‘precoprolites’(1, 2)
‘Precoprolites’ are preserved gut contents, regurgitates, or intestinal residues (Pollard 1990). Exceptional conditions are needed for soft stomach contents to be preserved, but they provide the best direct evidence for diet, especially if undigested (Pollard 1990). Stomach contents are rare in plesiosaurs (Sato and Tanabe 1998). One pliosaur specimen from the Lower Oxford Clay of Peterborough has teuthid cephalopod hooklets preserved in its gut region alongside sparse fish teeth and an indeterminate reptile tooth (Martill et al. 1991). The hooklets closely resemble those of the contemporaneous belemnite, Belemnoteuthis antiquus. Another specimen of a pliosaur, Pliosaurus brachyspondylus from the Kimmeridge Clay, also contains the remains of an armoured dinosaur (Taylor et al. 1993). A plesiosaur specimen from northern Japan preserves ammonoid jaw apparatus (possibly two taxa) concentrated in abundance in the stomach region (Sato and Tanabe 1998), and this provides the only known evidence that ammonoid cephalopods (ammonites) were consumed by plesiosaurs. The plesiosaur Umoonasaurus demoscyllus (AM F.99374) from Australia contains 17 vertebrae from an unidentified teleost fish in the gut region – direct evidence for a diet of bony fish in this individual (White et al. 2023).
Local and seasonal variations in the availability of prey is also a factor that can influence diet (Massare 1987, Martill et al. 1994), so stomach contents found in a few specimens may not be fully representative of what those species ate. It is possible that some of the stomach contents in large plesiosaurs have a secondary origin, i.e. from the stomachs of prey ingested by the plesiosaur, but this is unlikely.
Coprolites(1, 2)
Coprolites provide direct evidence for diet, but for which species? Coprolites are relatively common in Mesozoic marine strata and while we can be quite certain they belong to large marine reptiles, it usually isn’t possible to positively identify coprolites as being made specifically by plesiosaurs. So, coprolites must be used in association with criteria 2, 3 and 5 to determine ‘who did it?’. Reptile coprolites from mesozoic marine deposits, some of which may belong to plesiosaurs, are rich in calcium-phosphate (Pollard 1990) and often contain remains of fish and reptiles (Ford and O’Connor 2002).
Bite marks (1, 2)
Pliosaur bite marks are present on many bones, including a small isolated Kimmeridge Clay plesiosauroid propodial (fig 4) (Martill et al. 1994), and ichthyosaur vertebrae (Martill 1992). The shape, size, and spacing of the tooth marks may allow determination of the biter. For example, Pliosaurus has large distinctive trihedral teeth, so slightly triangular tooth marks can be confidently attributed to Pliosaurus.
Functional morphology of the skull (3)
The jaws are the primary structure for acquiring food so functional analysis of the jaws and dentition (teeth) is useful for determining diet (Pollard 1990). Evidence for twist-feeding in some pliosaurs comes from their strong triangular shaped skulls, deeply rooted large teeth, and expanded mandibular symphysis (Martill et al. 1994). These characteristics would resist torsional forces when rolling in the water. A detailed analysis of the functional morphology of the skull has been performed for two well-known pliosaurs, Rhomaleosaurus zetlandicus from the Lower Jurassic (Taylor 1992b) and Pliosaurus brachyspondylus from the Upper Jurassic (Taylor and Cruickshank 1993a). Rhomaleosaurus is interpreted as feeding on a wide range of prey and forcibly dismembering large prey. In P. brachyspondylus, the cranium is robust and the posterior teeth are unusually recurved (fig 5) to act as a ratchet to pull struggling prey into the mouth (Taylor and Cruickshank 1993a). Large postorbital openings in all plesiosaurs contained well-developed M. adductor mandibulae muscles to ensured a powerful bite (Massare 1988).

Plesiosaurs occur in a wide range of forms able to deal with prey of all sizes (Martill et al. 1994). The small-headed ‘plesiosauromorphs’ were unable to rip chunks from carcasses because their skulls were lightly built (Brown and Cruickshank 1994) with compressed mandibular rami and a weak mandibular symphysis, so they were unable to resist torsion (Cruickshank 1994b). Plesiosaur teeth were not used for chewing so the size of prey in these forms was directly limited by the size of their gullet (Massare 1988). The shape of the sclerotic ring suggests the eyes were flattened to assist with underwater vision (Lambert et al. 2001), and they were oriented upwards in many plesiosaurs. This suggests that they ambushed prey from below rather than from above. This contradicts a traditional interpretation for elasmosaurids using their necks to dart down at fish from above water (the ‘hunting platforms’ hypothesis) (Storrs 1993, Everhart 2002). Some pliosauroid eyes are positioned laterally (Massare 1988) (see Storrs and Taylor 1996), which suggests that they attacked prey on the same level in the water column. Although the function of the long neck is still unresolved (Martill et al. 1994, Storrs 1993, Noè et al. 2017) it probably allowed the plesiosaur to approach prey while keeping its body at a distance, to avoid being detected (Massare 1988).
Hearing and smell
Stapes (ear bones) are rarely preserved in plesiosaurs but where known, it is evident they were of no use in hearing (Storrs and Taylor 1996), at least in air (Lambert et al. 2001). An olfactory system (Cruickshank et al. 1991) (fig 8) has been suggested as a common adaptation in the Plesiosauria (Brown and Cruickshank 1994). The anteriorly placed internal nostrils have palatal grooves that may have helped channel water into them, with flow maintained by hydrodynamic pressure over the posteriorly placed external nares, caused by movement of the animal through the water. In this hypothesis, the water may have been ‘tasted’ during its passage through the nasal ducts by olfactory epithelia. However, a study by Buchy et al. (2006) questions the position of the internal nares, and proposes that they are actually located at the rear of the palate (the posterior interpterygoid vacuities).
Post-cranial functional morphology
Swimming capabilities have implication for diet (Robinson 1975, Massare 1988). Plesiosauroids and pliosauroids had different locomotory repertoires. pliosaurs probably ‘sprinted’ in short bursts using their relatively large flippers to take fast moving prey “by stealth rather than pursuit” (Martill 1992). Plesiosauroids generally have lower flipper aspect ratios and were more likely endurance swimmers (Robinson 1975). Massare (1988) came to similar conclusions based on the hydrodynamic properties of Mesozoic reptiles and calculated speeds of 2.3 m/sec (=slow ambush predator) for plesiosauroids and slightly faster (=pursuit predator) speeds for pliosauroids. For more information on locomotion in plesiosaurs visit the locomotion page.
Gastroliths (3, 5)
We have known since the turn of the 20th century that plesiosaurs and extinct crocodiles intentionally swallowed stones (see Williston 1893, Williston 1894, Williston 1904). The presence of gastroliths is common in plesiosaurs. Pollard (1990) noted that plesiosauroid stomach contents “usually contain gastroliths” (fig 9), and gastroliths are particularly common in elasmosaurids (see Everhart 2000, and Cicimurri and Everhart 2001). Gastroliths are rarer in pliosaurs (Storrs 1993, Sato and Tanabe 1998) but gastroliths are reported in one specimen cf. Liopleurodon sp. by Martill (1992), along with a fraction of sand, and isolated gastroliths are reported in Rhomaleosaurus (Smith 2007a). The difference may be genuine and could represent different functional regimes between pliosaurs and plesiosaurs (Storrs 1993).
What was the function of gastroliths in plesiosaurs? They may have has a role in buoyancy control (Chatterjee and Small 1989), this would be a less physiologically expensive way of attaining negative buoyancy than pachyostosis (Martill and Naish 2000). Recent work by Don Henderson (Henderson 2006) suggests that gastroliths have a role in stability within the water column rather than buoyancy control. Where present, gastroliths are usually found in small concentrations, although 100 are known for some elasmosaur specimens (Everhart 2002). The relative weight could be insignificant in large animals (Cicimurri and Everhart 2001, Everhart 2002), although it only takes a few grams to tip a balance. Gastroliths may alternatively have been used for grinding food in the gut. Gastroliths may have had a dual or even multi-purpose (Storrs 1993).
Analogy (5, 8)
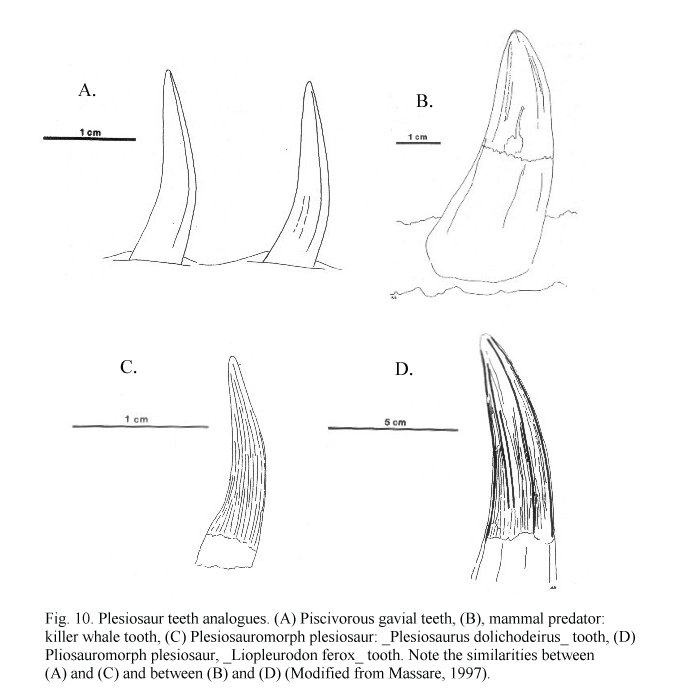
Plesiosaurs were air-breathing reptiles and must have surfaced frequently. They could have dived for food for limited periods of time, but such behaviour is difficult to determine for an extinct animal with no modern descendants or close relatives (homalogues). Analogy can be used to infer diet in marine reptiles. Martill et al. (1994) regarded several extant marine organisms including cetaceans, penguins and pinnipeds as analogous to plesiosaurs in many aspects. Massare (1987) presented a number of similarities between the teeth of marine reptile and modern large marine carnivores (fig 10). The teeth of the piscivorous gavial (Gavialis gangeticus) show similarities with plesiosauromorphs (fig 10. A and C), while the teeth of killer whales (Orcinus orcus) share many similarities with pliosauromorphs (fig 10. B and D) (Massare 1987). Killer whales eat large vertebrates, so pliosaurs probably did, too. Plesiosauroid teeth also interlock, another adaptation of piscivores (Benton 1990a). A ‘rushing upwards’ style of attack is inferred for large pliosauromorphs an analogy drawn from the modern great white shark (Carcharodon) (Martill and Naish 2000). If plesiosaurs were ectotherms (e.g. Chatterjee and Small 1989) then they would presumably be able to go for considerable periods of time without food.
Incomplete carcasses (2, 5)
Isolated bones and partial articulated skeletons are found in many Mesozoic deposits and may be indicative of twist-feeding and shake-feeding (Martill et al. 1994). The bones may have been dropped by large pliosaurs in the water column, although it is also possible they dropped from floating carcasses.
Predator or scavenger?
Evidence for scavenging of floating corpses comes from dinosaur remains (Taylor et al. 1993) and pterosaurs (Massare 1987) in stomach contents. Predation consists of several phases: (1) search, (2) capture, (3) penetration, (4) ingestion (= subjugation), (5) digestion, and (6) defecation (Brett 1990). Phases 1 to 3, in particular, have repercussions on the biology of the predatory organism. The following predation phases can be reasonably inferred from plesiosaur anatomy:
1. Search: Large eyes, olfactory system, locomotor adaptation.
2. Capture: Tooth and skull functional morphology, isolated bones (possibly dropped from prey), and incomplete but articulated skeletons (possible prey).
3. Penetration: Tooth and skull functional morphology: i.e. fast snapping muscular jaws and reinforcing pterygoid flanges.
Pre-ingestion breakage may provide direct ichnological (trace fossil) evidence for predatory behaviour, but diagnostic bite marks can represent both predation and scavenging. Predation can only be inferred if subsequent regrowth of the damaged prey (bone/shell) is present, as this would provide evidence for an unsuccessful attack. A pliosaur tooth embedded in the femur of a Metriorhynchus crocodylomorph from the Oxford Clay is on display in the Thinktank Brimingham Science Museum. The bone has regrown around the tooth, providing direct evidence of predatory behaviour in pliosaurs – the Metriorhynchus got away.
Ecological guilds
Since plesiosaurs are so diverse it is sensible to split the group into ecological guilds independent of their taxonomy. Massare (1987) divided large aquatic reptiles into groups based on their tooth form and placed the groups into triangular morphospace (fig 11). Of seven marine reptile ecological guilds, the Plesiosauria occupy three: ‘pierce’, ‘cut’, and ‘general’. Subsequently, a new guild has since been proposed.

Unusual plesiosaurs
The pliosaur Pachycostasaurus (fig 13) from the Oxford Clay or Peterborough, UK (Dawn 1997), does not fit neatly into any of the categories proposed by Massare (1987). It is regarded as a generalsistic feeder. Its pachyostotic ribs allowed it to traverse the seafloor, perhaps searching for benthic prey. Another unusual case is Aristonectes, which has hundreds of tiny delicate teeth (fig 14). An additional guild has been proposed to accommodate such oddities: a ‘trap guild’ (Chatterjee and Small 1989). This may be analogous to the extant crab eater seal, which has sieve-like teeth for capturing krill (Martill et al. 1994). Cryptoclidus also has similar teeth and shares its environment with the very common decapod crustacean (Mecochirus). This relationship fulfils criteria 2. It is unlikely than any plesiosaurs were strict suspension feeders in the sense of modern baleen whales (Colin and Janis 1997).

Conclusions
All plesiosaurs were predatory carnivores belonging to one or more of four ecological feeding guilds. Pliosaurs were top carnivores in their respective foodwebs (Martill 1992, Martill et al. 1994, Sato and Tanabe 1998). Many pliosaurs were pursuit predators of various sized prey, and opportunistic feeders in the ‘general’ and ‘cut’ guilds defined by Massare (1987). Twist feeding in large pliosaurs was also likely (Taylor 1992b).
The plesiosauroids such as Plesiosaurus were more specialised feeders (Maisch and Rücklin 2000) belonging to two guilds, the ‘pierce 1’ and ‘general’ guilds. Their teeth were used to pierce small soft-bodied prey such as fish and cephalopods (Massare 1987). Some taxa diversified from these feeding groups and were perhaps filter feeders (trap guild). Hard and soft-bodied cephalopods also formed part of the diet of all plesiosaurs. Plesiosaurs hunted visually and possibly had a directional sense of olfaction.
